照片/聲音
觀察者
mnold1描述
Mag. 100x (3), 400x (1,2,4,5)
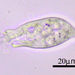
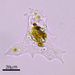
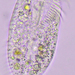
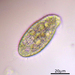
Large ciliate (though the anterior portion looks worm-like). Three specimens: A in photos 1&2, B and C in photos 3,4, & 5. All 3 specimens traveled exclusively in a semi-transparent, mucilaginous tube; this is especially apparent in the low-power photo, 3.
- After gently pushing aside the cover of lily pads, a pond side water sample was taken on 06/30/2022 using a 10µ dip net to enrich for microbes. Air temp. 81°F.
照片/聲音
什麼
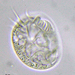
屬 Galeripora觀察者
crseaquist描述
Water sample taken from the edge of a stagnate freshwater playa on 2023-11-06 using a turkey baster.
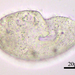
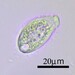
i. Diameter: 155 µm
ii. Aperture: 56 µm
iii. Height: 49 µm
iv. A/D: 0.36
照片/聲音
什麼
屬 Colpoda觀察者
crseaquist描述
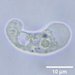
Gathered dry leaves on 2024-02-23 and stored in water.
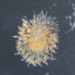
Two daughters emerging from a division cyst:
- Initial observation of cyst with another individual
- 2 minutes later
- 18 minutes later
- 77 minutes later
- 84 minutes later
- 140 minutes later
- 153 minutes later
- 154 minutes later 1st daughter emerges
- 155 minutes later
- 162 minutes later
- 163 minutes later 2nd daughter emerges
- 2nd daughter swims away
Video: https://youtu.be/vntikKDmSDE
照片/聲音
觀察者
crseaquist描述
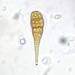
Gathered dry leaves on 2024-02-23 and stored in water.
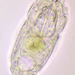
Here are some videos:
https://youtu.be/8DMTa0ZruLc
https://youtu.be/izC2XI3pRPY
https://youtu.be/1Q_9wdLj5pc
I think the videos seem to show it is an eruptive morphotype.
照片/聲音
觀察者
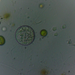
mnold1描述
Mag. 400x
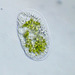
- A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
Very large specimen of A. conica. Siemensma notes a maximum dimension of ~100µ (for his and literature observations.) A large Arcella (up to 345µ wide) with a highly folded test is A. formosa , but its pie shape (highly folded and flattened) does not seem appropriate for the current specimen.
觀察者
pfau_tarleton描述
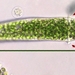
Numerous of these spherical colonies in the ephemeral pond.
Video of this colony: https://youtu.be/pIXz9PIPkWE
A much higher quality video of something similar: https://www.sciencephoto.com/media/921392/view/sinantherina-colonial-rotifer-light-microscopy-footage
照片/聲音
觀察者
crseaquist描述
Water sample taken from the edge of a freshwater playa on 2024-01-28 using a turkey baster.
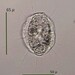
Length: 90 µm
Width: 45 µm
Trophi: 32 µm
Toes: 14 µm
Ratios: L/TL: 6.4
Two eyespots. Type C virgate trophi.
- Dorsal view shows the two eyespots and trophus.
- Lateral view showing toes.
- Another lateral view.
- Remainder of images show various views of the trophus.
照片/聲音
什麼
屬 Mayorella觀察者
crseaquist描述
Water sample taken from the edge of a freshwater playa on 2024-01-28 using a turkey baster.
照片/聲音
觀察者
crseaquist描述
Water sample taken from the edge of a freshwater playa on 2024-01-28 using a turkey baster.
照片/聲音
觀察者
davidfbird描述
From a sample of periphyton scraped from the rocks at the falls. Cell length 65 µm, dorsoventrally
flattened with concave oral area on ventral surface, slightly asymmetric at anterior end. One dorsal contractile vacuole and a posterior oval macronucleus. On the left-hand side of the large oral opening is a very large undulating membrane. You can see it well in the last gif, which is slowed down 8X.
I put a longer video excerpt on facebook: https://www.facebook.com/100070304167055/videos/1735270383617100/.
照片/聲音
什麼
屬 Thuricola觀察者
mnold1描述
Mag. 200x (1,4); 400X (2,3)
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Thuricola, a loricate peritrich ciliate. Lorica has a valve flap that must be pushed open to allow the zooids to feed. The lorica is round-bottomed and stalkless. Images in the video, https://youtu.be/om-CaI8zPlk, suggest there may be a vertical indentation at the aperture of the lorica.
照片/聲音
什麼
門 Cercozoa觀察者
crseaquist描述
Water sample taken from the edge of a freshwater playa on 2023-12-07 using a turkey baster.
The images show the flattened ovoid shape of the test with a lens like cross section. No rod like spines are visible. There do appear to be xenosomes. The third image seems to show a CV and nucleus. It seems to be between 30 and 40 microns long.
Videos:
https://youtu.be/rNY33wLxCXo
https://youtu.be/BZW5JillJPo
照片/聲音
觀察者
davidfbird描述
From the tychoplankton, collected by net beside the bridge across the stream. The zooids are about 60-70 µm in diameter, and the open peristomial lip is about 100 µm wide. The stalks are 500-600 µm long, extended. Presumably V. campanula. One of the shots is of the cells stained with a fluorescent nucleic acid stain (not entirely specific to nucleic acids as you can see). The last gif is slowed 60 X, trying to capture the moment of contraction.
照片/聲音
什麼
門 Cercozoa觀察者
crseaquist描述
Water sample taken from the edge of a freshwater playa on 2023-12-07 using a turkey baster.
Video: https://youtu.be/f22Nib6qYbg
照片/聲音
觀察者
davidfbird描述
These Fe-Mn deposits formed within 24 h on the surface film of a sample from the marsh. I can't help but find them fascinating. The single, or double, tiny cells inside each nest makes its living taking electrons from dissolved, reduced iron and manganese ions, which rusts them and makes them insoluble. But how does the cell avoid getting iron stuck on its exterior membrane surface? And why is the distribution of cells not random? They look like penguin nests that are all separated by at least a mutual neck length.
Some of the cells are attached to one end of the filaments of another sort of iron bacterium, like comets with a tail. Too often to be coincidental, I think.
Nests of iron bacteria also form on the surface of a dead leaf, so they don't need an air-water interface, as long as there is some oxygen in the water.
照片/聲音
觀察者
zookanthos描述
First gif 6x speed. Followed it for about 5 minutes and didn't notice any changes in shape or behavior. The posterior end had a few very small fingerlike projections that didn't get captured well in the photos.
照片/聲音
觀察者
ian_gardiner描述
Habitat small, temporary, grassland pool.
Image 1 - female, ventral view with eggs and two attached spermatophores.
Image 2 - male, ventral view.
Image 3 - male 5th pair of legs, posterior view, showing i) inner and distal processes on left exopod 2 forming a pincer-like structure, ii) spiniform process on outer distal corner of right basis.
Image 4 - male right first antenna, showing short, curved, spinous process at distal end of antepenultimate segment.
Image 5 - female posterior dorsum showing elongate outer sensilla on metasomal wings.
Image 6 - female 5th pair of legs.
照片/聲音
觀察者
davidfbird描述
On a small piece of decayed vegetation from the marsh. The microscope field at 1000x is 200 µm wide, and this creature is larger than that when extended. The third gif is slo-mo so that you can almost catch a glimpse of the 'head'.
照片/聲音
觀察者
crseaquist描述
Water sample taken from the edge of a stagnate freshwater playa on 2023-11-06 using a turkey baster.
照片/聲音
什麼
病毒 (界 Viruses)觀察者
davidfbird描述
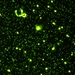
Bacteriophages and bacteria from saline ponds and lakes of southern Saskatchewan. A volume of fixed sample water, about a tenth of a ml in these environments, is concentrated by filtration onto a exceedingly fine filter, and stained with a fluorescent dye that lights up when docked on nucleic acids (both DNA and RNA). This makes the viruses and bacteria (and phytoplankton) visible for counting in an epifluorescence microscope. Even the smallest viruses become visible and countable.
But isn't the resolution of the light microscope a factor of ten too large to see viruses? The trick works according to the same principles that allow you to see and count stars that are 16000 light years away: because they are sources of bright light compared to the background. The stars appear substantial, but their real diameter is almost infinitely smaller than the size that they appear to your eye.
In these photos, bacteria appear relatively large. In the first photo, I count roughly 50 bacteria. But you can see that the viruses that are parasites of bacteria, or bacteriophages, are very much more abundant. There are between 25 and 30 bacteriophages for every bacterium. This has probably been the relative abundance of bacteria and their phages in these environments for 100s of millions or even billions of years. How this equilibrium is maintained is pretty much a mystery for the moment. More below (tomorrow).
照片/聲音
觀察者
crseaquist描述
Water sample taken from the edge of a stagnate freshwater playa on 2023-11-06 using a turkey baster.
什麼
屬 Alternaria觀察者
kschnei描述
Found in semi-stagnant water (not much else seen) from creek near entrance gate. Janai S. suggested this was a testate amoeba and could be genus Certesella (I had no clue what this was at all...).
照片/聲音
觀察者
davidfbird描述
Lac Gilbert is a headwater lake in a valley, which protects it from the wind. It has a relatively deep hole (12 m) at one end that allows the bottom water to go anoxic over the course of the summer. The stability of the water column means that distinct bands of different communities develop in layers in the water, and also on the sediments. For instance, above the anaerobic zone that starts at 9 m depth, there is a band of Hydra on the sediments that feeds on migrating zooplankton.
The surface water is pristine and low in nutrients, so phytoplankton abundance is low, allowing light to penetrate deep into the lake. Photosynthetic pigments increase greatly in the anoxic layer, where large populations of anoxygenic photosynthetic bacteria appear. Just above the oxygen-free zone, the sediments develop a dense mat of spiral cyanobacteria, Arthrospira jenneri. Their identity is given away by the thickness of the filament relative to coil length. A. jenneri, like all cyanobacteria, is facultatively anaerobic. That means it can grow in a zone that is bathed in the nutrients released by anaerobic processes.
These are images from both the water column and the sediment mat. Some are transmitted light and some are by epifluorescence. Some are unstained and some are stained with a fluorescent dye (DAPI) that makes nucleic acids fluoresce light blue. Arthrospira is blue-green by transmitted light, and red by epifluorescence. The red of epifluorescence signals the presence of chlorophyll. Sediment samples will have bright yellow particles. These are granules of polyphosphate that are stored by bacteria and that glow yellow when stained by DAPI. The deep water column samples, without DAPI, contain cyanobacteria, and also photosythetic picoplankton (red dots) that are probably photosynthetic bacteria.
The filament labelled Oscillatoria in one of the photos is orange because it contains a fluorescent auxiliary pigment, phycoerythrin. It might be another member of the Oscillatoriales, maybe Planktothrix, but there is no large Planktothrix layer in the lake.
照片/聲音
什麼
病毒 (界 Viruses)觀察者
davidfbird描述
I thought it would be fun to start putting some unusual records of free-living microbial interactions, made using different sorts of microscopy. This one is from the hypolimnion of a dystrophic lake, Lac Croche, at the UdeM field station. What is going in this photo captured by electron microscope? I think the large cell is a photosynthetic bacterium, I don't remember which sort. I suspect that it is not happy. Its coat is covered with bacteriophage particles. Look at the blow-up and you will see their icosahedral head shape (which looks hexagonal in profile). They're tiny, and tailless, so I'm thinking RNA viruses (Orthornavirae). We don't know if the viruses are coming or leaving - probably the latter. Some other kind of bacteria have docked on the photosynthetic bacterium's side. They might be friends or they might be foes, we shouldn't jump to conclusions. But it sure looks like some kind of savanna-level takedown.
照片/聲音
觀察者
peptolab描述
Aspidisca leptapsis Fresenius, 1865 from the coarse sand sulfidic intertidal benthos of marine estuary Acabonac Harbor at Louse Point launching ramp. Imaged in Nomarski DIC on Olympus BH2 using SPlan 40x objective plus variable phone camera cropping on Samsung Galaxy S9+.
The cells measure from 66-80 um in length. There are four dorsal ribs on the rigid pellicle. There is a prominent left peristomial spur adjacent to the posterior AZM and a smaller left lateral anterior spur. The posterior cell margin shows 3 or 4 subtle serrations or spurs. There are six transverse cirri with the rightmost one being split into two at the base. There are seven strong fronto-ventral cirri and a single weak one. These characters are congruent with the description of the species by Song and Wilbert (1).
From Song and Wilbert 1997:
"Size of our populations in vivo about 60-80x40-50 um, body elliptical with snout-shaped anterior end; broadest in or behind mid-body, right margin convex, left almost straight, anteriorly broadly rounded; dorso-ventrally highly flattened (ca. 1:2.5-3). Outline associated with indentation or protrusion of cell, one lateral spur subapically located, which is usually smaller than prostomial one even inconspicuous in some specimens. Additionally, several (4-6) posterior spurs on border of caudal portion, which make the cell usually rugged (q.v. Borror 1968; Dragesco 1960; Kahl 1932; Tuffrau 1964). Ventral surface generally flattened, on dorsal always 4 dominant ridges. Buccal area with 1 distinct thorn-like projection (prostomial spur) on left subcaudally, which covers the AZM2. Pellicle rigid. Cytoplasm hyaline. Contractile vacuole right of median, at level of adoral zone of membranelles. Macronucleus C-shaped, with many spherical nucleoli (ca. 1-2 um). Micronuclei several in number (usually 3-5), always located in depressions of macronucleus. Movement crawling on substrate, quite fast compared with other congeners. When disturbed, always attached to substrate firmly for quite a while. Ciliary pattern stable. Frontoventral cirri rather stiff and strong, cilia of 7 large ones about 12-15 um long; one small, satellite-like cirrus close to right-most (posteriormost) cirrus, which is easy to be overlooked (q.v. Agamaliev 1967). Transverse cirri usually strong, tightly arranged in oblique row, base of right-most cirrus always splitting (but mostly imperfectly) into closely spaced 2-3 ones or even more, which are in vivo clearly recognizable. Cilia of transverse cirri about 15-20 um long. Anterior portion of adoral zone (AZM1) containing 7-8 membranelles, located in deep concave. Posterior part (AZM2) significantly long, as usual beneath lid-like prostomial spur. Paroral membrane (PM) small, difficult to observe. Pharyngeal fibres curved anteriorly. Constant 4 dorsal kineties with tightly spaced basal body pairs. Very characteristically, both basal bodies ciliated with rossete-shaped granules at bases of cilia. Each kinety along dorsal ridge and generally extending from end to end of cell (yet 2 central rows may be slightly shortened at posterior end)" (1).
"As we observed both from newly collected samples and in culture, the dorsal ridges and spurs in caudal region, which form the rugged body outline, could vary from less to very conspicuous. Since those characters are so variable and depend so much on description of the observers, we appraise these as weak characters for species separation. Alike is the number of transverse cirri: since the left-most one is only partly divided (the bases are closely connected), the number of those cirri might depend in a large scale on the quality of impregnation. Due to these factors, we suggest that forms with 5-8 transverse cirri, with or without appearance rugged body outline but similar in all other morphological (in vivo and at infraciliature level), biological (habitat, behaviour) and morphometric features should be considered as the same species. Based on the reasons stated above, we synonymize with Aspidisca leptaspis the following species (see; Syn) simply because they show basically no differences but the number of transverse cirri and "absence" of spurs at caudal end of cell" (1).
The redescription of A. leptapsis by Li et al (2010) lists the following synonyms laid out by Song and Wilbert (1997): "Aspidisca leptaspis Fresenius, 1865 Syn. A. sedigita Quennerstedt, 1867; A. crenata Fabre-Domergue, 1885; A. sedigita sensu Dragesco, 1960; A. lyncaster sensu Tuffrau, 1964; A. baltica sensu Borror, 1968; A. psammobiotica Burkovsky, 1970; A. lyncaster sensu Fleury et al., 1968". They depict the subtle posterior serrations (also called spines- see figure from their paper included here) (2).
- Morphological Investigations on Some Free Living Ciliates (Protozoa, Ciliophora) from China Sea with Description of a New Hypotrichous Genus, Hemigastrostyla nov. gen. Weibo Song, Norbert Wilbert. Archiv für Protistenkunde Volume 148, Issue 4, December 1997, Pages 413-444
- Morphological Redescriptions of Aspidisca magna Kahl, 1932 and A. leptaspis Fresenius, 1865 (Ciliophora, Euplotida), with Notes on Ontogenesis in A. magna. Liqiong LI, Qianqian ZHANG, Khaled A. S. AL-RASHEID, Choon Bong KWON, Mann Kyoon SHIN. Acta Protozool. (2010) 49: 327–337
照片/聲音
什麼
屬 Oxytricha觀察者
crseaquist描述
From an aquarium at home where I pour old samples and the water from washed microscope slides.
L: 50 µm
W: 22 µm
什麼
複雜生殖器類 (下目 Entelegynae)觀察者
zookanthos描述
Cup 17 tree. Silkhenge structure. Apparently the spiders involved in making these still haven't been determined.
照片/聲音
觀察者
mnold1描述
Mag. 100x (1-3), 400x (4-6)
- A pond-side water sample (retentate) was taken using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Rotifer. Two thorn-like spines at the top of the foot. Long toes. Looks similar to Trichotria tetractis as seen here https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Trichotria%20tetractis.html and as observed by @paul_norwood here on iNaturalist https://www.inaturalist.org/observations/95064795.
For a video of this speciment: https://youtu.be/pIN2rfAvreE.
照片/聲音
觀察者
bdstaylor描述
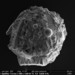
An amoeba that builds its shell from found materials (xenosomes). This one incorporates diatoms, the spherical cysts of golden algae and tiny grains of sand.
Collected in the Mer Bleue Bog conservation area, air-dried on conductive tape and images in a scanning electron microscope at the Canadian Museum of Nature.
照片/聲音
什麼
屬 Torymus觀察者
jeongyoo描述
Bedeguaris species group. Either T. vesiculi or T. pachypsyllae
Ovipositing inside a hackberry leaf gall.
照片/聲音
什麼
屬 Tetmemena觀察者
crseaquist描述
Water sample taken from the edge of a stagnate freshwater playa on 2023-08-27 using a turkey baster.
Model for labels:
https://en.wikipedia.org/wiki/Stylonychia#/media/File:Stylonychia_mytilus.png
Thank you to Bruce Taylor for help with these labels.
什麼
蚊科 (科 Culicidae)觀察者
mnold1描述
Mag. 100x
- A pond-side water sample was taken on 07/31/2023 using a 10µ dip net to enrich for microbes. Air temp. 75°F.
Flatworm? Insect larva? Looks like it has retractable landing gear! :o) (i.e., the 4 objects surrounding the central, vesiculate oval object).
照片/聲音
觀察者
bdstaylor描述
Likely a Leptopharynx costatus with zoochlorellae.
Like other microthoracids, it is equipped with extrusomes. When exploded, these have four arms at the tip (a character of the family Microthoracidae, per Omar & Foissner, 2012, link below). The last two images show discharged extrusomes.
I looked at two specimens closely (the first is shown in images 1-3, the second in images 4 and 5). Both were a bit over 50 μm in length, and resemble those of L. bromeliophilus (as recorded in Omar & Foissner, 2011, link below).
照片/聲音
什麼
屬 Ophrydium觀察者
mnold1描述
Mag. 400x
- A pond-side water sample was taken on 07/31/2023 using a 10µ dip net to enrich for microbes. Air temp. 75°F.
Unsure about the ID. I think these are telotrochs (unattached, swimming phase of a sessilida spicies) of a vorticella-like critter. WRONG! SEE COMMENTS. They were relatively numerous in the water sample. All were filled with zoochlorellae. Evident when extended, they have a squarish posterior and tapered anterior that ends in a mouth-like structure or the remnant of a stalk. Near the anterior portion of some, but not all specimens, there is a ring of beating cilia (highlighted by red arrows in the first image).
View video here https://youtu.be/shyrjySczo0.
照片/聲音
觀察者
elytrid描述
Host was Mississippi Kite, Ictinia mississippiensis. Laemobothrion maximum is apparently a known ectoparasite of Ictinia mississippiensis. https://phthiraptera.myspecies.info/category/avian/aves/falconiformes/accipitridae/ictinia/ictinia-mississippiensis
This is my first time seeing bird lice in person and these look really interesting.
Big thanks to Melissa for saving these two individuals for me. Definitely something I never expected to have in my collection.
照片/聲音
觀察者
mnold1描述
Mag. 400x
- plankton-drag samples were collected by the 2023 Desmid Project team of @karolina et al at Victory Basin sample site 4. (This is a non-desmid observation.)
Small Actinosphaerum? Appears to have an outer layer of large vesicles (characteristic of this genus of heliozoa). The overall shape is ovoid and it appears flattened (judging from the images of its surface, shown in the composite image). The axonemes seem sparse; perhaps this critter is in travel rather than eating mode (as relected in its plankton-drag capture). The interior is fill with interesting structures... none of which can I identify. (I had hoped to see multiple nuclei embedded near the periphery.) For info on Actinosphaerium see https://arcella.nl/genus-actinosphaerium/.
照片/聲音
觀察者
kljinsitka描述
153µ semi-vertical plankton tow
Covered in what could be a stalked ciliate protozoa.
see:
https://www.aphotomarine.com/ciliate_protozoa_copepod_09-09-17.html.
short video clip I took
https://youtube.com/shorts/6fYR6jW3KYc?feature=share
照片/聲音
什麼
原藻界 (界 Chromista)觀察者
brettjackson描述
Quick manual focus stack of a few images. Seems like it is composed of equilateral triangles, kind of resembling a regular icosahedron.
Putting ID as Chromista for now to include radiolarians, centric diatoms, dinoflagellate cysts, etc.
照片/聲音
觀察者
peptolab描述
Metopus spiculatus from the sulfidic fine sand superficial intertidal benthos of marine estuary Acabonac Harbor. Imaged in Nomarski DIC on Olympus BH2 using SPlan 40x objective plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 84 um in length. The Latin adjective “spiculatus” refers to the distinctive, beak-like anterior protrusion which is present in this specimen. The needle-like intracytoplasmic structures were not visualized. Sample collected Jan 2023- organism observed 7-2-2023.
Marine form about 55–100 × 25–40 μm in vivo. Body shape oblong to ovoid. Preoral dome extremely compressed, distal end curved and tapered into a conspicuous beak-like structure. Posterior body tapered sharply into an about 25 μm long tail. Cell surface covered by a layer of rod-shaped ectosymbionts arranged perpendicularly except for most of the preoral dome and tail zone. Cytoplasm filled with needle-like intracytoplasmic structures, aggregate in the anterior part. Elongate ellipsoidal macronucleus centrally located below the preoral dome and spherical micronucleus. On average, 21 somatic kineites including 5 preoral dome kineties. Perizonal stripe rows 1 and 2 forming false kineties. The adoral zone consisted of about 20 membranelles, occupying about 30% of body length. Paroral membrane double-rowed with one about twice as long as the other. Sulfide-rich sediments in an intertidal zone in Liujiawan, Rizhao (119°26′, N35°17′), China.
M. spiculatus sp. n. can be distinguished from all other Metopus by the following characteristics: (i) marine habitat, (ii) rod-shaped ectosymbionts, (iii) a beak-like structure at the preoral dome end, (iv) a posterior body that tapers into a tail, (v) an in vivo size of 75–100 × 30–40 μm, (vi) 17–22 adoral membranelles and 19–25 somatic kineties, and (vii) needle-like intracytoplasmic structures. The new species resembles M. vestitus Kahl, 1932 (Figure 6G) in most features except the distinct beak-like preoral dome end (present vs. lacking). Similar to this new species, M. caudatus, Tropidoatractus acuminatus and Tropidoatractus spinosus are medium-sized, and have an oblong body and one acute tail, but all of them lack ectosymbionts, which are considered as an important feature for species identification (Esteban et al., 1995; Rotterová et al., 2018; Li et al., 2021a). In addition, M. rostratus Kahl, 1932 and Tropidoatractus levanderi Rotterová et al., 2018 also possess a beak-like preoral dome end, but both lack a long tail and conspicuous ectosymbionts as shown in our form (Kahl, 1932; Foissner, 2016a; Rotterová et al., 2018).
New Contribution to the Diversity of the Anaerobic Genus Metopus (Ciliophora, Armophorea), With Descriptions of Three New Marine Species
Wenbao Zhuang, Ran Li, Xiaochen Feng, Saleh A. Al-Farraj and Xiaozhong Hu. Front. Mar. Sci., 26 May 2022. Sec. Marine Evolutionary Biology, Biogeography and Species Diversity. Volume 9 - 2022 | https://doi.org/10.3389/fmars.2022.884834. https://www.frontiersin.org/articles/10.3389/fmars.2022.884834/full
照片/聲音
什麼
屬 Brachionus觀察者
crseaquist描述
Water sample taken from a stock tank of freshwater on 2023-06-23 using a turkey baster.
Images:
- Mother with egg (female)
- Anterior spines of mother
- Tail of mother
- Hatching of the egg start
- Egg cracking
- Emergence
- Mother and Daughter
- Daughter
- Daughter’s posterior
- Daughter’s front (from ventral side)
- Location
Video of egg hatching: https://youtu.be/-0KXoNNq2Xc
照片/聲音
觀察者
mnold1描述
Mag. 400x
Euglenoid with grit/sand-covered body, and a cytostome surrounded by a flared collar with a distinct rim. It uses a single flagellum for locomotion. It feeds on detritus and small algae. In the linked video you will see the creature worm its way through a mound of detritus, https://youtu.be/PUfNi0aoE5U. There were instances when it appeared to be foraging/feeding; the flared anterior moving over a surface like a handheld vacuum. :o) A very similar creature (but only in swimming rather than feeding mode) was observed from a different, local water-body https://www.inaturalist.org/observations/107162369.
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
照片/聲音
觀察者
mnold1描述
Mag. 100x
Rotifer. Constantly on the move; could not keep up at 400x. Head region sports a transparent collar or shield; making this critter look like it's wearing a Flash Gordon bubble helmet :o) https://dyn1.heritagestatic.com/lf?set=path%5B1%2F8%2F8%2F5%2F3%2F18853171%5D%2Csizedata%5B850x600%5D&call=url%5Bfile%3Aproduct.chain%5D. This specimen looks similar to images of Squatinella lamellaris f. mutica recorded here http://www.rotifera.hausdernatur.at/Species/Index/2445?AddScansGrid-page=1.
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
照片/聲音
觀察者
bdstaylor描述
A few more specimens of Ileonema dispar, the type species of the genus, which, as far as I know, has not been recorded since it was discovered by Stokes in 1884. It is differentiated from I. simplex by its flagellar process, which, in the species Stokes describes, is in two distinct parts, thick at the base and finely filamentous at the distal end (Stokes, 1884; Penard, 1922; Kahl, 1930). This is reflected in the name of the species, "dispar" (=unequal). Stokes also describes the species as having two contractile vacuoles in the posterior, an arrangement that would be extremely odd for a trachelophyllid ciliate. In several specimens, I observed some compartmentalization in the posterior vesicle, which could easily be read as two separate vacuoles adjacent to the "anal pore." See image #4.
The flagellar process is retractable, and its appearance is quite variable. The division of this organelle into two distinct parts--which I did not see in every specimen--strikes me as a weak character. As I noted in my previous observation, I think there is some reason to think that I. simplex should be considered a junior synonym of I. dispar (or possibly a variant, or sub-species).
I caught one in fission, and have included two images of the dividers.
I also watched an individual eating a huge clump of debris containing living and dead algae. This observation, and the contents of the cytoplasm in other individuals, lead me to think the critter lives by consuming small round green algae.
照片/聲音
什麼
屬 Brachionus觀察者
crseaquist描述
Water sample taken from the stagnate edge of a freshwater playa on 2023-06-21 using a turkey baster.
照片/聲音
觀察者
crseaquist描述
Water sample taken from the stagnate edge of a freshwater playa on 2023-06-21 using a turkey baster.
照片/聲音
觀察者
peptolab描述
Saprodinium dentatum from the decomposing leaf matter at the edge of my long-neglected garden pond imaged in Nomarski DIC using Olympus BH2 using SPlan 40x objective plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 66 um in length, body discoid, laterally compressed. Left side with 3 spines the 2 posterior-most two having ciliary rows. Right side with three smaller spines devoid of ciliary rows, the dorsal spine having ciliary row and the large ventral tooth. One macronucleus. Oral opening and adoral zone of membranelles the middle of the ventral side. Perizonal ciliary row very long on right side. Contractile vacuole below adorale zone of membranelles.
"Saprodinium dentatum is a highly asymmetrical cell with extremely reduced somatic ciliature and a number of site-specific spines. The cell shape resembles a laterally compressed helmet of a Roman soldier. The anterior and the dorsal edges of the cell are wedge-shaped, while the ventral and the posterior sides of the cell are wider. Both the length (measured from the anterior to the posterior pole) and depth (measured from the dorsal to the ventral side) of the cell are of about 60 um. Widest point, measured from the right lateral side to the left lateral side in the lower ventral area of the cell, is 15 um. The right side of the cell is dominated by the frontal band consisting of the ciliated anterior segments of five somatic kineties. The long axis of the frontal band is at a right angle to the anterior-posterior axis of the cell. The left side of the cell bears a long dorsal kinety to the left of the dorsal keel. Moreover, the left lateral view shows part of the oral ciliature that is hidden in a complex oral cavity. The oral apparatus lies near the left side of the cell where the body wall is thin and transport. The opening of the oral cavity to the outside is close to the prominent ventrocaudal spine that bears three caudal spine kineties.
The majority of the spines are located around the posterior end of the cell and therefore are called spines. Moreover, a so-called oral spine on the ventral ridge is just above the opening of the oral cavity (this "tooth" close to the mouth led to the name "tooth-mouthed" odontostomes (Corliss 1979), while the "comb-mouthed" ctenostomes had their name from the comb-like array of the adoral membranelles (Kahl 1932)). Finally, a frontal spine is a continuation of the dorsal keel; the dorsal keel is the anterior compressed part of the dorsal ridge. Living cells of S. dentatum are easily identified by their characteristic locomotion and transparency. Cells either swim in a jerky motion or creep with help from the frontal band cilia so that the right side of the cell is orientated toward the substratum. Closer light microscopical observations show that S. dentatum has usually 1-3 macronuclei (12 um in diameter) and one micronucleus (3 um in diameter) located in the posterior part of the cell. Because of the cell's transparency, eight adoral membranelles can be seen in the biggest part of the buccal cavity (actual complexity of the oral apparatus is only seen in electron micrographs). A conspicuous feature in the cell's anterior is an accumulation of strongly refractile spherical bodies called lithosomes (Andre & Faure-Fremiet 1967) because of their inorganic nature. Lithosomes consist of alternating electron-dense and electron-transparent concentric layers of unknown chemical composition; the bigger lithosomes may cause serious difficulties during thin sectioning. Saprodinium dentatum has no typical mitochondria. The cytoplasm is crowded with endobiontic bacteria; many of them seemingly undergoing cell division. Two bacteria of different size can be distinguished; the big ones look similar to the large Gram-negative bacteria found in the heterotrich ciliate Metopus striatus, and the small ones may correspond to the small-sized Gram-positive bacteria in the same ciliate" (1).
- Hans-Gunther Schrenk and Christian F. Bardele; The Fine Structure of Saprodinium dentatum Lauterborn, 1908 as a Representative of the Odontostomatida (Ciliophora). J.Protozool. 38(3):278-293, 1991
照片/聲音
觀察者
peptolab描述
A ciliated swarmer of the suctorian Sphaerophrya magna Maupas, 1881 in my long-neglected garden pond filled with rotting leaves. I previously showed the feeding behavior (https://www.inaturalist.org/observations/162692722). This swarmer measures 30 um in diameter and shows an actively cycling contractile vacuole and large central round macronucleus.
S. magna from this site measure from 37.5 to 50 um in diameter. Imaged in Nomarski DIC using Olympus BH2 under SPlan 40x objective plus variable phone cropping on Samsung Galaxy S9+.
Sphaerophrya magna Maupas, 1881 Spherical; numerous tentacles of different length; nucleus spheroid; standing fresh water with decaying vegetation. Measurements
About 50 um in diameter.
照片/聲音
觀察者
crseaquist描述
Water sample (freshwater) was taken on 2023-04-29 using a turkey baster.
I am sorry that these images are horrible; however, I think they give enough information for me to communicate what I was seeing:
A collared flagellate with a single apical flagella. The flagella appears very long, ~5C.L. I don’t think I see any traces of a lorica or theca. There appear to be projections on the posterior. The organism’s flagella is so long and it whips it about with such vigor that the whole organism moves back and forth. Images 1, 4 show the long flagella; images 1, 2, 3, 6 show the microvilli collar; images 2, 3, 5, 6 show what might be posterior projection; the last image, a gif, captures the movement.
My impression was that I was observing something like what is shown here: https://www.youtube.com/watch?v=mV01Kruf9Ig
照片/聲音
什麼
屬 Aspidisca觀察者
crseaquist描述
Water sample (freshwater) was taken on 2023-04-09 using a turkey baster.
照片/聲音
什麼
屬 Bicosoeca觀察者
mnold1描述
Mag. 400x
Bicosoeca sp., I think. This is a filter-feeding flagellate housed in a transparent, vase-like lorica, and has an anterior flagellum (for feeding and transportation) and an posterior flagellum that anchors to the base of the lorica and allows the cell to retract into the lorica when necessary. These Bicosoeca are living epiphytically on a colony of diatoms, Asterionella formosa.
For 2 videos see: https://youtu.be/GjPdiDE_VOY and https://youtu.be/vCKK5l6yxFM.
For a fabulous a video and photos of Bicosoeca, see the observation recorded by iNatter closterium_mysterium, https://www.inaturalist.org/observations/145952656#activity_comment_7f982c87-a61d-4cd0-88b0-cf67d9969e39.
- A water sample was taken on 1/24/2023, from the shore of Rosemond Lake , using a 10µ dip net to enrich for microbes. Air temp. 42°F.
照片/聲音
觀察者
crseaquist描述
Water sample (freshwater) was taken on 2023-04-29 using a turkey baster.
I was so surprised to see the stalk contract, which is the subject of the 2nd and 3rd images.
照片/聲音
什麼
螳水蠅屬 (屬 Ochthera)觀察者
matthias22描述
On mud at edge of pond. There were a lot of them. I was photographing the male when it suddenly mounted the female. Later they started to copulate. When the flies walk around they wave their fore legs (see first two pictures). It looks strange and I don't know what the purpose of this behaviour is. Collected for the Royal Alberta Museum.
照片/聲音
照片/聲音
照片/聲音
觀察者
mnold1描述
Mag. 400x
Filled with Zoochlorellae, like Acanthocystis turfacea and A. pernardi, both found locally. However. this specimen is very different in that it is covered with loose, elliptical scales (5-10µ in length). I do not see any spine-scales. The long axipodea are very granular and are, occasionally, piled high from the base with elliptical scales (40-50µ high!). The elliptical scales form a disorganizes, 10µ thick layer enveloping the entire protoplasm. The best candidate (by my eye) for an ID, at Siemensma's Microworld, is Raphidocystis ambigua https://arcella.nl/raphidocystis-ambigua/. A 2nd possibility is R. symmetrica https://arcella.nl/raphidocystis-symmetrica/, but the current specimen is much larger than those recorded by Siemensma. No reference to Zoochrorella is made in describing either taxon. I could not find a match among the Acanthocystis.
- A water sample was taken on 5/8/2023, from the shore of Lantern Hill Pond, using a 10µ dip net to enrich for microbes. Air temp. 73°F.
觀察者
harsiparker描述
Photo achieved by gently scooping some water into a container while on-site at a small freshwater pond. After being photographed, the larva was quickly returned to the same portion of the pond where it was found. I always do my best to minimize how much I disturb the critters I wish to photograph -- my aim is to leave them unharmed and in the exact same microhabitat/location where I discovered them.
照片/聲音
觀察者
peptolab地點
私人的描述
Bresslaua vorax Kahl, 1931
In an attempt to find tardigrades in the abundant lichen growing on my untreated deck, I instead grew out from the lichens soaking in water lots of colpodiids. First there were a lot of small colpodids and soon larger carnivorous Colpodidae, Bresslaua vorax, appeared and began feasting on their smaller cousins. It is amazing how the vestibule has evolved to capture and consume their preferred meal, the much smaller bacteriovore colpodids.
Bresslaua exhibits dimorphism, ie the species occurs in 2 morphological forms depending upon nature of food supply. The smaller (microstome) form grows when micro-algae and bacteria are the food source; this transforms into the larger (macrostome) form when fed upon ciliates. The shape of the huge cytopharyngeal vestibule fits the contours of the smaller colpodid population like a glove. The small colpodids almost seem to volunteer to be eaten by their much larger cousins. I show here two different individuals of different sizes enjoying their meals. I think the larger one first depicted is a large trophont. As Bruce Taylor writes: "The species is common, and notoriously gluttonous known to cannibalize its microstome brothers, which might even raise the possibility that you have one species here!.
Imaged in Nomarski DIC using Olympus BH2S under SPlan 40x objective plus variable phone cropping on Samsung S9+.
Bresslaua Kahl, 1931
Class Kinetofragminophora: Subclass Vestibulifera: Order Colpodida
Suborder Colpodina Foissner, 1978: Family Colpodidae Ehrenberg, 1838
Bresslaua vorax Kahl, 1931
Dimorphic; microstome form, pyriform to reniform, plastic, with a latero-ventral invagination, 35-100 um by 16-50 um; macrostome form, oval in dorsal view; rigid with convex dorsal and flattened ventral surface, up to 250 um long; the prominent cleft in the anterior quarter forms the anterior margin of vestibular opening; one macronucleus and one micronucleus; encystment.
Key to the Colpodidae From: Biol Fertil Soils (1990) 9:110-118 Kuehneltiella terricola gen. nov., sp. nov. a carnivorous ciliate (Protozoa, Ciliophora) from a sandy soil in Australia * W. Foissner. http://www.wfoissner.at/data_prot/Foissner_1990_110-118.pdf
Foissner writes: The most common soil ciliates belong to the class Colpodea, and Foissner (1987a) has designated the soil ciliate community as "Colpodetea". The evolutionary success of the Colpodea in terrestrial biotopes depends on their r-selected survival strategy, especially on their capability to produce four or more offspring in reproductive cysts. Within the family Colpodidae, two feeding strategies have been evolved. Members of the large genus Colpoda (about 30 reliable species) feed mainly on bacteria, and are comparatively small-sized species with a funnel-shaped mouth. These species obviously profit from the high number of microbes present in terrestrial biotopes. The three other genera of this family, Bresslaua, Krassniggia, and Kuehneltiella gen. nov., each consisting of only a few large species, have a huge oral apparatus occupying the anterior half of the cell. These species are rapacious carnivores, consuming the production of bacteria-feeding ciliates and flagellates. Lynn (1979) suggested that carnivorous colpodids evolved from bacteria-feeding ones".
照片/聲音
觀察者
crseaquist描述
Water sample (freshwater) was taken on 2023-04-29 using a turkey baster.
I can hardly believe this is a Foram since this is freshwater. The sample includes dirt at the bottom of water column.
照片/聲音
什麼
榕小蜂 (科 Agaonidae)觀察者
tobiashays描述
From cultivated Ficus (microcarpa?) synconium.
From same fig:
https://www.inaturalist.org/observations/159028375
https://www.inaturalist.org/observations/158994180
照片/聲音
什麼
屬 Sycoryctes觀察者
tobiashays描述
From cultivated Ficus (microcarpa?) synconium.
Male from same fig:
https://www.inaturalist.org/observations/158994180
觀察者
jgw_atx描述
(tentative ID)
On underside of Ficus carica leaves at night.
Collected a series of specimens for Martin Hauser